
Detection and treatment of apnea with Kalinix
27 Sep 2021

Obstructive sleep apnea/hypopnea syndrome (OSAHS) is a very common disease, affecting a large percentage of the population. It is estimated that between 9% and 26% of adults, and up to 3% of children, suffer from it.
Obstructive sleep apnea occurs when there is an obstruction in the airway during sleep. After which breathing returns with a snore or snort. Sufferers stop breathing for periods longer than 10 seconds, even up to 2 minutes, throughout the night.
These respiratory arrests cause alterations in heart rate, tiredness, drowsiness and irritability, which can affect the course of normal life and even lead to traffic and work-related accidents.
The conventional treatment recommended for OSAHS is CPAP: a mask connected to an air pump that administers constant pressure to the patient during the night, to prevent the throat from "closing". More than a third of patients do not tolerate the CPAP mask and abandon the treatment.
There are also other less popular treatments, such as mandibular augmentation devices (MAD) and surgical treatments.
Is there an alternative method for the treatment of apnea?
For those affected by apnea and hypopnea that do not admit conventional treatments, the new Kalinix device offers an effective, non-invasive and even permanent alternative to existing OSAHS treatments currently on the market.
Kalinix is an electromedical apnea and hypopnea detection and treatment device that detects and treats respiratory arrest in real time.
How does Kalinix work?
Kalinix's technology for the detection and treatment of apnea is based on a smart, wearable, mobile phone-sized, multi-sensor and self-adaptive device.
Thanks to its algorithms, it performs predictive detection and real-time treatment of respiratory arrest by means of specific stimulation waves.
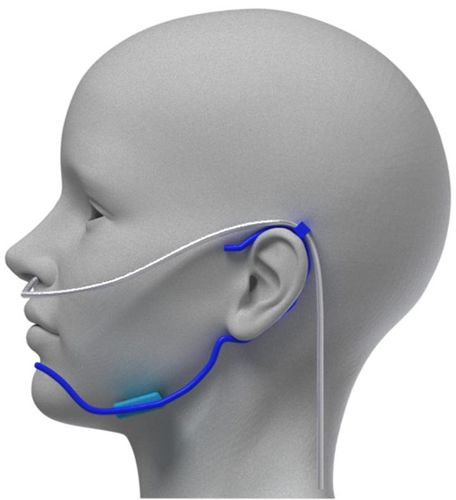
Therapeutic treatment
Kalinix offers a potential improvement solution for OSAHS thanks to its therapeutic treatment.
- Provides a toning stimulation of the muscles associated with the respiratory tract.
- Improves its elastography, i.e. it tones the involved musculature, preventing airway obstruction.
- Applies sequences of stimulation waves when the patient is awake.
- Currently in its third phase of trials.
Previous studies carried out at the Pneumology Department of the Hospital Clínico San Carlos directed by Prof. Dr. José Luís Álvarez Sala Walther, the Hospital Teknon in Barcelona and the collaboration of Dr. Ernesto Delgado Cidranes of the Hospital Vithas La Milagrosa, led to the hypothesis that, with the toning of the muscles associated with the physiopathology of OSAHS, there could be an improvement in the main parameters of sleep.
Real-time treatment
Kalinix is a non-invasive solution which minimizes side effects and increases patient acceptance.
- Predicts OSAHS events in real time.
- Provides correction through superficial electrical impulses that open the airway.
- Gives a treatment that is imperceptible to the patient.
- Does not alter sleep structure.
- Self-adjusts the intensity and frequency of the electrical impulse, adapting to the episode and patient characteristics.
- Allows storing the night's information and automatically analyzes the evolution of OSAHS.

Diagnosis
Kalinix allows to perform a simplified sleep study.
- Allows to perform home polygraphs.
- Detects and records apnea and hypopnea episodes in real time.
- Is capable of self-diagnosis, providing an immediate result, facilitating the work of the specialist.
- Simplifies implantation, enabling placement by the patient himself.
- Minimizes costs and waiting lists.
Kalinix is currently in the validation phase of clinical trials at the Hospital Clínico San Carlos and in the process of obtaining the CE marking.
To keep up to date with all the news and advances in the launch of Kalinix you can follow us on our social networks:
Related News

Developing and testing Kalinix for the treatment of apnea
In recent years, Torytrans has promoted the building of a multidisciplinary team, made up of renowned doctors and an experienced group of engineers,…
Read more

Torytrans develops a portable system to combat sleep apnea
A sleep apnea solution that provides immediate relief by improving all the patient's respiratory parameters in real time, without discomfort and in a…
Read more
